how to draw molecular orbital diagrams for polyatomic molecules
Taking the internuclear axis as the z -axis we have. Assigning those to the group of hydrogens.
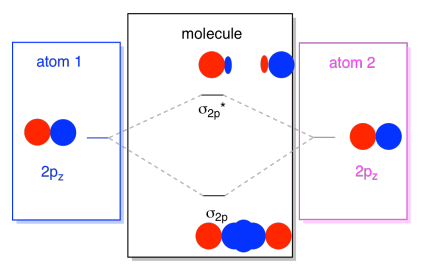
3 3 4 Assembling A Complete Mo Diagram Chemistry Libretexts
Molecular Orbital Diagrams Simplified By Megan A Lim Medium 1 Ligands and lone pairs act as if they repel each other.

. However with more atoms computers are required to calculate how the atomic. Roussel MOs for polyatomic molecules January 8 20201023. Bond order ½ Bonding es - Antibonding es A B 1σg 2σu 1s 1s 2s 2s 2p 2p 3σg 4σu 1πu 5σg 2πg 6σu.
Bond order can be calculated by the formula. Walsh diagrams Walshs rule A molecule adopts the structure that best stabilises the HOMO. Label each level with σ σ π π In the construction of molecular orbital diagrams for heteronuclear molecules the bonding mos are shown closed to electronegative atoms while.
Then well build the MO diagram from scratch. Because the and ˇ orbitals arise from independent LCAOs we can build the ˇ orbital energy diagram independently of the orbitals. AO1s AO1s σ1s σ 1s strong head-on overlap AO2s AO2s σ2s σ 2s strong head-on overlap AO2px AO2px π2px π 2px weak sidelong overlap AO2py AO2py π2py π 2py weak sidelong overlap AO2pz AO2pz σ2pz σ 2pz strong head-on overlap.
Can be accommodated in the metal d orbitals. Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. The concept of a molecular orbital is readily extended to provide a description of the electronic structure of a polyatomic molecule.
Symmetry adapted linear combinations salcs we need salcs aka group orbitals to draw molecular orbital mo diagrams of polyatomic molecules. From this diagram calculate the bond order for O 2. In chemistry resonance also called mesomerism is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures or forms also variously known as resonance structures or canonical structures into a resonance hybrid or hybrid structure in valence bond theoryit has particular value for.
If omitted is set to 0. C Calculate the average CC bond order based on. And this should make sense because no is isoelectronic with co which has a bond order of 3.
Decide if the molecule is homonuclear of heteronuclear. Draw out the mo diagram and label in the. The overall steps well be following are.
Three approaches to bonding in diatomicmolecules 1Lewis structures 2Valence bond theory 3Molecular orbital theory Chapter 5 Bonding in polyatomic molecules 2 Orbital hybridization - sp Hybrid orbitals generated by mixing the characters of atomic orbitals 2 1 ψsp_hybrid ψ2s ψ2px 2 1 ψsp_hybrid ψ2s ψ2px 3. Fill the mos with electrons. This orbital is called sigma-star σ and is less stable.
In chemistry molecular orbital MO theory is a method for describing the electronic structure of molecules using quantum mechanics. In most cases this means that molecules tend to adopt the geometry that maximises the. If the HOMO is unperturbed by the structural change under consideration then the occupied MO lying closest to it governs the geometric preference.
In this video qualitative MO diagrams will be generated for simple polyatomic molecules water ammonia and difluorocarbene using so-called ligand group o. Finding the point group. Draw the molecular orbital diagram for the oxygen molecule O 2.
Atomic orbitals are representations for where electrons are likely to. 6 Best Free Molecular Modeling Software For Windows. How to draw molecular orbital diagrams for polyatomic molecules Atomic Orbitals.
For molecules with nice Lewis diagrams octet rule satis ed that have multiple bonds the frontier orbitals are very frequently ˇ orbitals. Finding the reducible representation based on how the hydrogen atoms behave. 8-11 indicates that the 1b 1 orbital does resemble a 2 p atomic orbital on oxygen but one which is polarized into the molecule by the field of the protons.
Bond order can be calculated by the formula. To a first approximation the 1b 1 molecular orbital will be simply a 2 py atomic orbital on the oxygen perpendicular to the plane of the molecule. You have to start filling the orbitals from those with lowest energy to those with higher energy.
MO diagram of homonuclear diatomic molecules Filling the resulting MOs of homonuclear diatomic molecules with electrons leads to the following results. Determining which orbitals are compatible. Specify the length and temperature of the heating run and cooling phases of a dynamics run.
Reducing the reducible representation to its irreducible representations.

Molecular Orbital Theory For Homonuclear Diatomic Molecules Pt 3 Youtube
8 7 Molecular Orbitals For Polyatomic Molecules Chemistry Libretexts

Ocn Lewis Structure How To Draw The Lewis Structure For Ocn Drawings Tech Company Logos Lewis

8 4 Molecular Orbital Theory Chemistry
8 7 Molecular Orbitals For Polyatomic Molecules Chemistry Libretexts
Molecular Orbitals Molecular Orbitals For Polyatomic Molecules

Molecular Orbital Mo Diagram Of Trihydrogen Ion Chemical Bonding Molecular Structures Youtube
8 7 Molecular Orbitals For Polyatomic Molecules Chemistry Libretexts
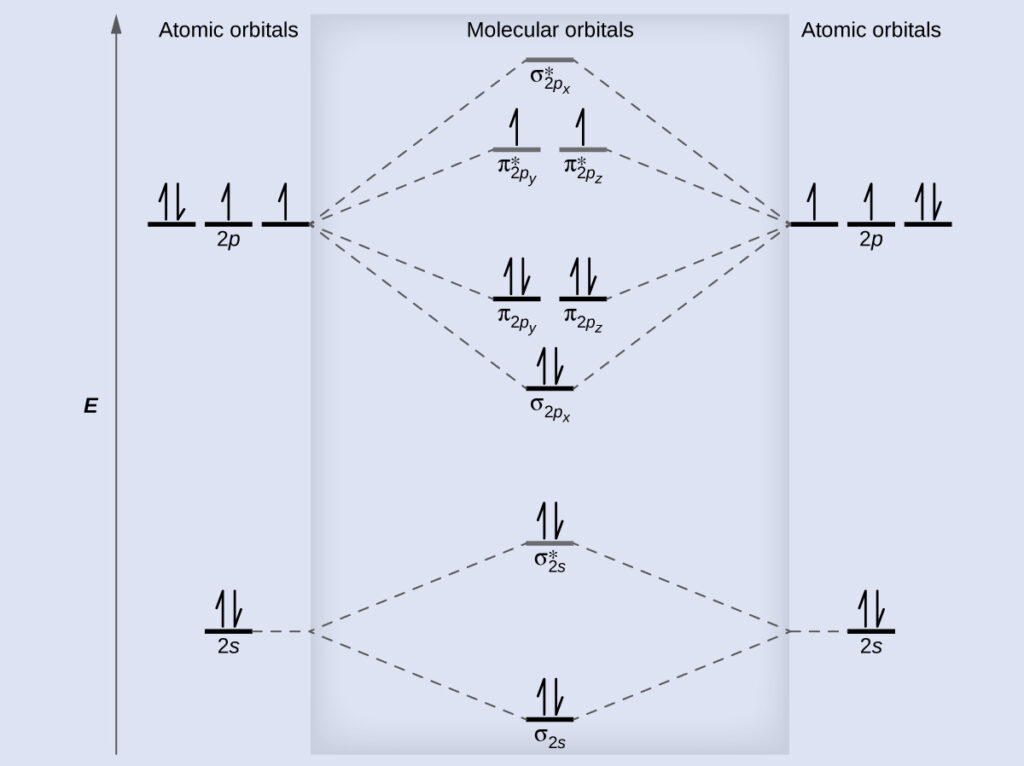
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Chem Textbook

Chemical Bonding Molecular Orbitals Of Polyatomic Species Britannica
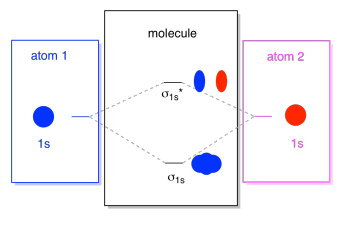
3 3 4 Assembling A Complete Mo Diagram Chemistry Libretexts

8 4 Molecular Orbital Theory Chemistry

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube

5 4 Larger Polyatomic Molecules Chemistry Libretexts

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube

Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube

Molecular Orbital Diagram Of O2 F2 And Ne2 Molecules Youtube

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube